Neural and hormonal mechanisms underlying species and sex differences
Regulation of Sex Differences in Communication Signals by Gonadal Steroid Hormones
Both EOD frequency and chirping are sexually dimorphic in many species of electric fish, but the magnitude and the direction of sex differences varies across species. In brown ghost knifefish (A. leptorhynchus), males have higher EOD frequency and chirp 10-20 times more often than females. In black ghost knifefish (A. albifrons), males have lower EOD frequency than females, and chirping is much less sexually dimorphic (Dunlap et al. 1998; Kolodziejski et al. 2005). Sex differences in EOD frequency and chirping are regulated by hormones like 11-ketotestosterone (11-KT) and estradiol. The effects of these hormones vary between species in parallel with species differences in sexual dimorphism. 11-KT masculinizes EOD frequency in both A. leptorhynchus and A. albifrons, but does so by raising EOD frequency in A. leptorhynchus, but lowering EOD frequency in A. albifrons (Dunlap et al. 1998). The effects of androgens and estrogens on EOD frequency are mediated largely by effects of these hormones on the firing rates of neurons in the pacemaker nucleus and electromotor neurons in the spinal cord (Schaefer and Zakon 1997).
We study how steroid hormones affect sex and species differences in electrocommunication behavior by using several techniques:
(1) Treating different species and populations of fish with androgens and estrogens to correlate hormone effects on EOD frequency and chirping with the sexual dimorphism of these signals.
(2) Measuring levels of hormones in relation to sex, reproductive condition, social dominance, and electrocommunication behavior in different species.
(3) Studying species- and population-level variation in the expression of hormone receptors in brain regions that control the EOD and chirping.
(4) Combining hormone treatments with electrophysiology to study the effects of androgens and estrogens on the firing patterns and excitability of the neurons that control the EOD and chirping.
Neuromodulators and Chirping
To understand how sex differences in the brain have evolved to regulate sex differences in behavior, we study the neural control of chirping in different species of electric fish. Chirping is controlled by a dedicated brain region, the prepacemaker nucleus (PPn) (Click here for background information on the neural circuits that control the EOD and chirping).
Substance P is a peptide best known for its role in modulating the sensation of pain. Substance P is broadly distributed in the brain, however, and it influences many aspects of behavior and physiology, including mood, sleep, anxiety, motor rhythms, nausea, sexual behavior, and circadian rhythms. In electric fish, substance P also regulates chirping. The PPn is richly innvervated by substance P-containing fibers and terminals, and infusions of substance P increase the propensity to chirp and modify the structure of chirps (Weld et al. 1991; Weld and Maler, 1992). The expression of substance P in the PPn is highly sexually dimorphic and is associated with sex differences in chirping. In brown ghost knifefish (A. leptorhynchus), males chirp more than females, produce high-frequency chirps that females almost never produce, and have much more substance P in the PPn than females. Substance P innervation of the PPn is also highly sexually dimorphic in black ghost knifefish (A. albifrons; Kolodziejski, Nelson, and Smith, 2005). This finding is interesting, because unlike in A. leptorhynchus, the amount of chirping does not differ between the sexes in A. albifrons. In both A. albifrons and A. leptorhynchus, however, the structure of chirps is sexually dimorphic. These findings led us to hypothesize that sex differences in substance P in the PPn regulate sex differences in the structure of chirps, but not necessarily in the amount of chirping.
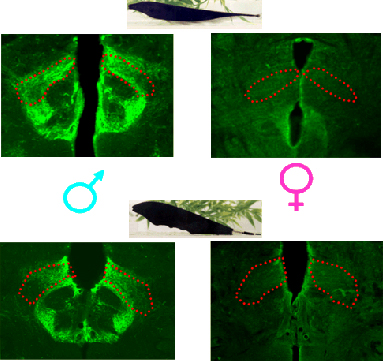 |
Sex differences in the expression of substance P in the prepacemaker nucleus (PPn) in A. leptorhynchus and A. albifrons. Brain sections containing the PPn (outlined in red) from males (left) and females (right) in brown ghost knifefish (top, A. leptorhynchus), and black ghost knifefish (bottom, A. albifrons). Substance P immunofluorescence is green. Note that males in both species have abundant substance P-containing fibers in the PPn, whereas there is almost no substance P immunoflourescence in the female PPn. Based on Kolodziejski et al. (2005). |
Another neuromodulator that influences chirping is serotonin. Serotonin modulates many behaviors including feeding, anxiety, motor rhythms, and aggression. Chirps are often produced during aggressive interactions between fish, and we are interested in testing the hypothesis that serotonin in the PPn modulates within- and between-sex variation in chirping. We recently found sex differences in the amount of serotonin in the PPn of brown ghost knifefish (Telgkamp, Combs, and Smith, 2007). The sex difference in the expression of serotonin in the PPn was in the opposite direction as the sex difference in substance P. Females had nearly twice as much serotonin in the PPn as males do. These results are consistent with previous results showing that females chirp less than males and that serotonin inhibits chirping (Maler and Ellis 1987). Thus, greater serotonin release in the PPn may suppress chirping in females.
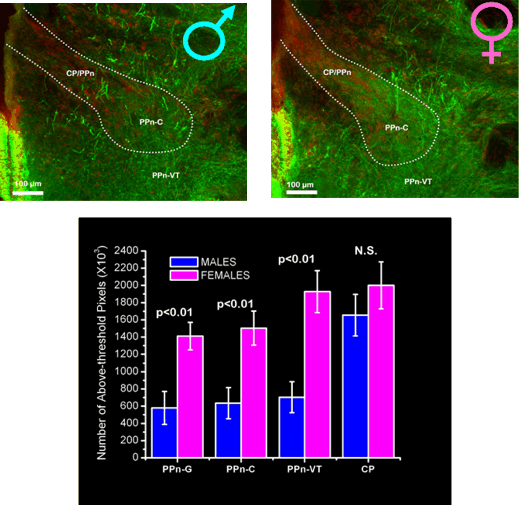 |
Sex differences in serotonin expression in the PPn of brown ghost knifefish (A. leptorhynchus). The PPn is outlined in white. Serotonin immunoreactivity (green) in the PPn of male (top left) and female (top right) brown ghost knifefish. Note the greater number of serotonin-containing fibers and terminals in the female PPn. (Bottom) Serotonin immunoreactivity in different subdivisions of the PPn. Females had significantly more serotonin in parts of the PPn that control chirping. Based on Telgkamp et al. (2007). |
In addition, we found an intriguing relationship between serotonin expression in the PPn and EOD frequency. In brown ghosts, EOD frequency is not only sexually dimorphic, but can also indicate social rank. Dominant fish of both sexes tend to have higher EOD frequencies than subordinate fish. We found that females with higher EOD frequencies (and that were thus likely to be dominant) had less serotonin in the PPn than presumably subordinate females that had lower EOD frequencies. Our results suggest that serotonin in the PPn may modulate both sex differences in chirping and differences in chirping between dominant and subordinate fish. We are currently conducting experiments to understand the mechanisms by which serotonin and social environment regulate chirping.
We use several techniques to investigate how neuromodulators regulate chirping:
(1) Immunohistochemistry to study species-, sex-, and individual-level variation in the distribution of neuromodulators in the PPn in relation to variation in chirping behavior.
(2) Recording chirping in response to playback stimuli before and after treating fish with agonists and antagonists of neuromodator receptors.
Neurophysiology of the Prepacemaker Nucleus and Chirping
Our laboratory developed an in vitro slice preparation of the PPn that allows us to make electrophysiological recordings from neurons in this brain region. We are using this preparation to address several questions:
(1) What is the intrinsic circuitry of the PPn, and how does it generate the command signal for chirps?
(2) How do neuromodulators like substance P and serotonin influence the excitability of neurons in the PPn and how do these effects on neuronal excitability relate to their effects on chirping?
(3) Are there sex differences in the physiological properties of neurons in the PPn and/or in the effects of neuromodulators on these neurons that contribute to sex differences in chirping?
(4) Do species differences in the effects of neuromodulators on the excitability of the PPn underlie species differences in the sexual dimorphism of chirp rate and/or structure?
